The Lateral Flow Test
The lateral flow test is a paper-based medical device for the qualitative detection of the presence or absence of target analytes in samples such as whole blood, plasma, serum, or fingertip blood. The lateral flow tests are easy to operate, affordable, and usually show results in 5-20 minutes.
The development and operating costs of lateral flow devices are relatively low compared to other diagnostic methods like molecular diagnosis. The lateral flow tests are widely used in hospitals, clinics, clinical laboratories.
Lateral flow assays can be used for the quick detection of cancer marker, cardiac marker, drug of abuse, pregnancy test, and infectious disease
And common sample types include urine, saliva, sweat, serum, plasma, whole blood, and other fluids such as fingertip blood.
The Principal
The principle of lateral flow immunoassays was first described in 1960, and the first at-home pregnancy test was launched in 1988. Since then, the lateral flow technology has been utilized to develop a large variety of applications for the clinical diagnosis and drug testing industry.
Lateral flow assays use the same technology as ELISA. These tests utilize the capillary force, which moves the liquids along a paper pad with reactive molecules that show a visual positive or negative result.
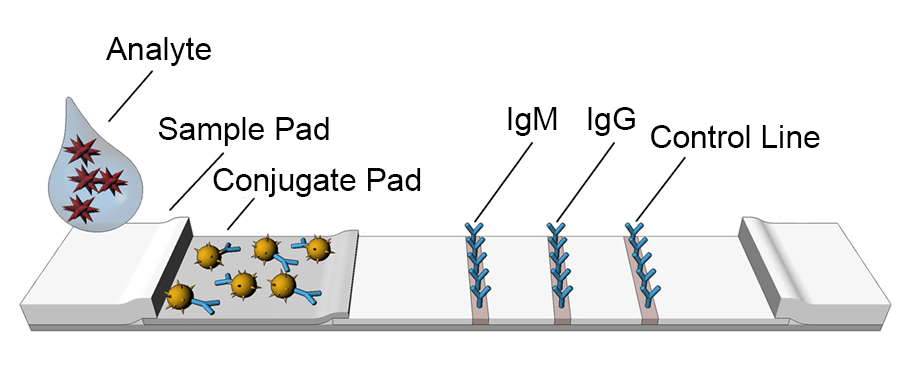
The COVID-19 Antibody Rapid Test Kit, for example, once the test sample is added into the sample pad on a lateral flow device, the sample flows through a filter film. If the test sample contains target analytes, it can combine with colloidal gold-labeled antigen to form a complex, which is captured by antibody coated with a colored band. The presence of colored bands indicates a positive result of the target analyte. Most of the lateral flow assays contain a control line, which works as a procedural control to inform enough samples were added into loading well. The absence of a colored control line indicates an invalid test result.
Result Interpretation
When conjugated antibodies accumulate at the immobilized test and control lines, the appearance of visible lines supplies for easy assessment of test results. In the case of gold nanosphere labels, the most commonly used labels, the visible lines are red in appearance and need no development procedure. The shade of red is decided by the gold nanoparticle dimensions, and strength is a factor of the sum of conjugated antibody bound at the immobilized lines.
The formation of a red line at the test line indicates a positive result (i.e., the existence of the target analyte). The intensity will depend on the quantity of target analyte from the sample. A quality control line must appear to indicate there was enough sample or dilution buffer added to the lateral flow test device.
Lateral Flow Assays in Daily Life
Lateral Flow Assays have a range of significant applications such as environmental contamination evaluation and point of care diagnostics. Probably the most well-known lateral flow assay application is the pregnancy tests, which detects human chorionic gonadotropin (hCG) in urine samples. When a woman becomes pregnant, the human chorionic gonadotropin (hCG) hormone is generated almost immediately. So the qualitative detection of hCG can be used to test if someone is pregnant.
Thanks to the properties and design of gold nanoparticles, and other labels, lateral flow assays have become a beneficial tool in several instances of calnical diagnostic applications and serve an essential role in healthcare and everyday life.

