COVID-19 Antigen Rapid Test Kit
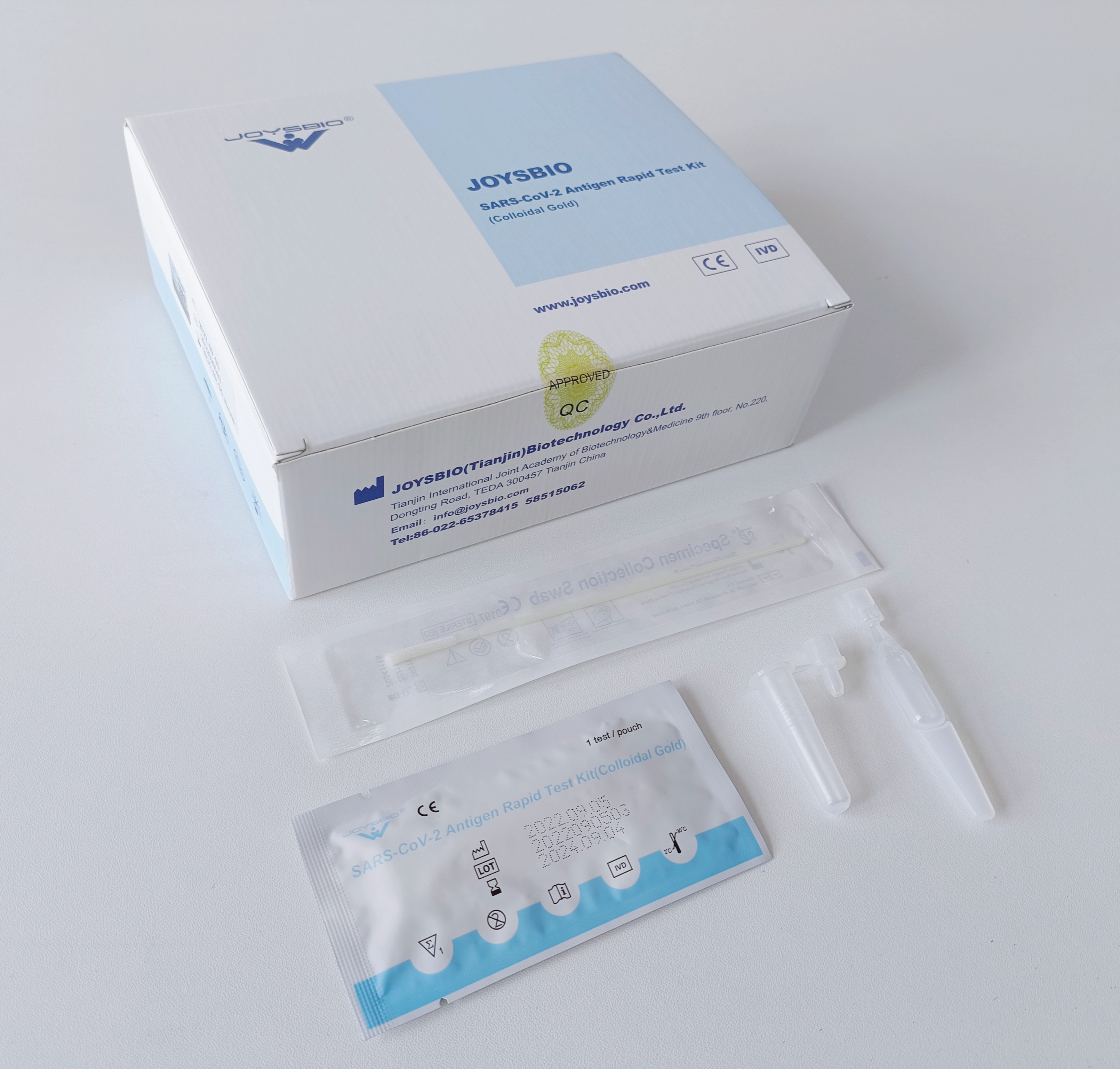
Coronavirus Antigen Rapid Test Kit
In June 2020, JOYSBIO Biotechnology proudly released a new COVID-19 Antigen Rapid Test Kit (Colloidal Gold). The new coronavirus antigen test kit is a lateral flow immunoassay for the qualitative detection of SARS-COV-2 antigen (nucleocapsid protein) in upper respiratory samples with nasal swabs or saliva during the acute phase of infection. An uncut sheet format is available.
Features
- 15-minute rapid detection
- Easy-to-operate coronavirus antigen test
- Less-invasive nasal (NS) swab sample collection
Performance Characteristics
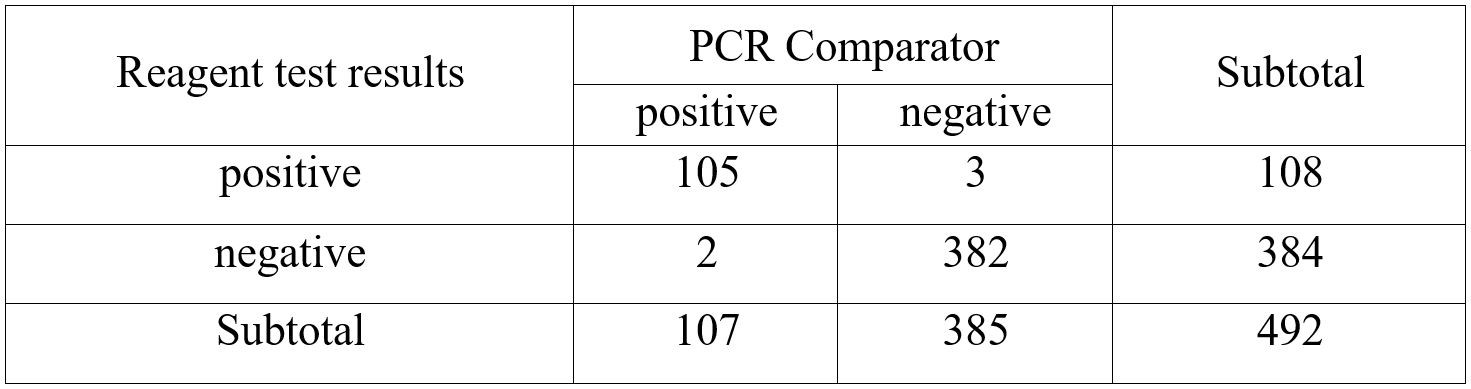
JOYSBIO’s coronavirus Ag test kit was independently evaluated at Centro Diagnostico Delta S.r.l. in Italy between October 2020 and January 2021. A total of 107 positive specimens were tested with JOYSBIO’s COVID-19 Antigen Rapid Test Kit. These specimens were collected from patients who are suspected of COVID-19 with nasal swabs. The coronavirus antigen test kit’s sensitivity and specificity are compared against a CE-IVD marked RT-PCR test kit. This clinical evaluation is conducted under the assumption that SARS-CoV is no longer spreading in the community.
According to the clinical analysis of 492 samples, the detection sensitivity is 98.13%, and the specificity is 99.22%.
- Positive Percent Agreement (PPA) = 105/107 (98.13%) (95%CI: 93.4%~99.8%)
- Negative Percent Agreement (NPA) = 382/385 (99.22%) (95%CI:97.7%~99.8%)
- Accuracy = (105+382)/492×100%=98.98%
- Kappa = 2×(105×382-3×2)/(108×385+107 ×384) = 0.97>0.5
The limit of detection (LOD) of this product is 1.6 x 102 TCID50/mL, calculated through a gradient dilution method.
Related Scientific Publications
- Five Antigen Tests for SARS-CoV-2: Virus Viability Matters
- Lateral flow antigen tests can sensitively detect live cultured virus of the SARS-CoV-2 B1.1.7 lineage
- Impaired performance of SARS-CoV-2 antigen-detecting rapid tests at elevated temperatures
- Limited specificity of SARS-CoV-2 antigen-detecting rapid diagnostic tests at low temperatures
COVID-19 Antigen Rapid Test Principle
The coronavirus antigen rapid test kit is a lateral flow assay that qualitatively detects the presence of nucleocapsid (N) protein in upper respiratory samples (nasal swabs). This lateral flow assay is designed with the sandwich immunoassay format. When the specimen is added onto the sample pad of a test cassette, coronavirus N protein binds with colloidal gold-labeled SARS-CoV-2 N protein antibody to form an antibody-antigen (Ab-Ag) complex. The Ab-Ag complex is captured by SARS-CoV-2 N protein antibody (Rabbit monoclonal antibody) when migrating to the test line under capillary action. A red-colored band will appear on the test line, which indicates the specimen is COVID-19 nucleocapsid protein positive. No color band will appear on the test line if the specimen does not contain any coronavirus antigen (N protein), or the antigen level is below detection limit.
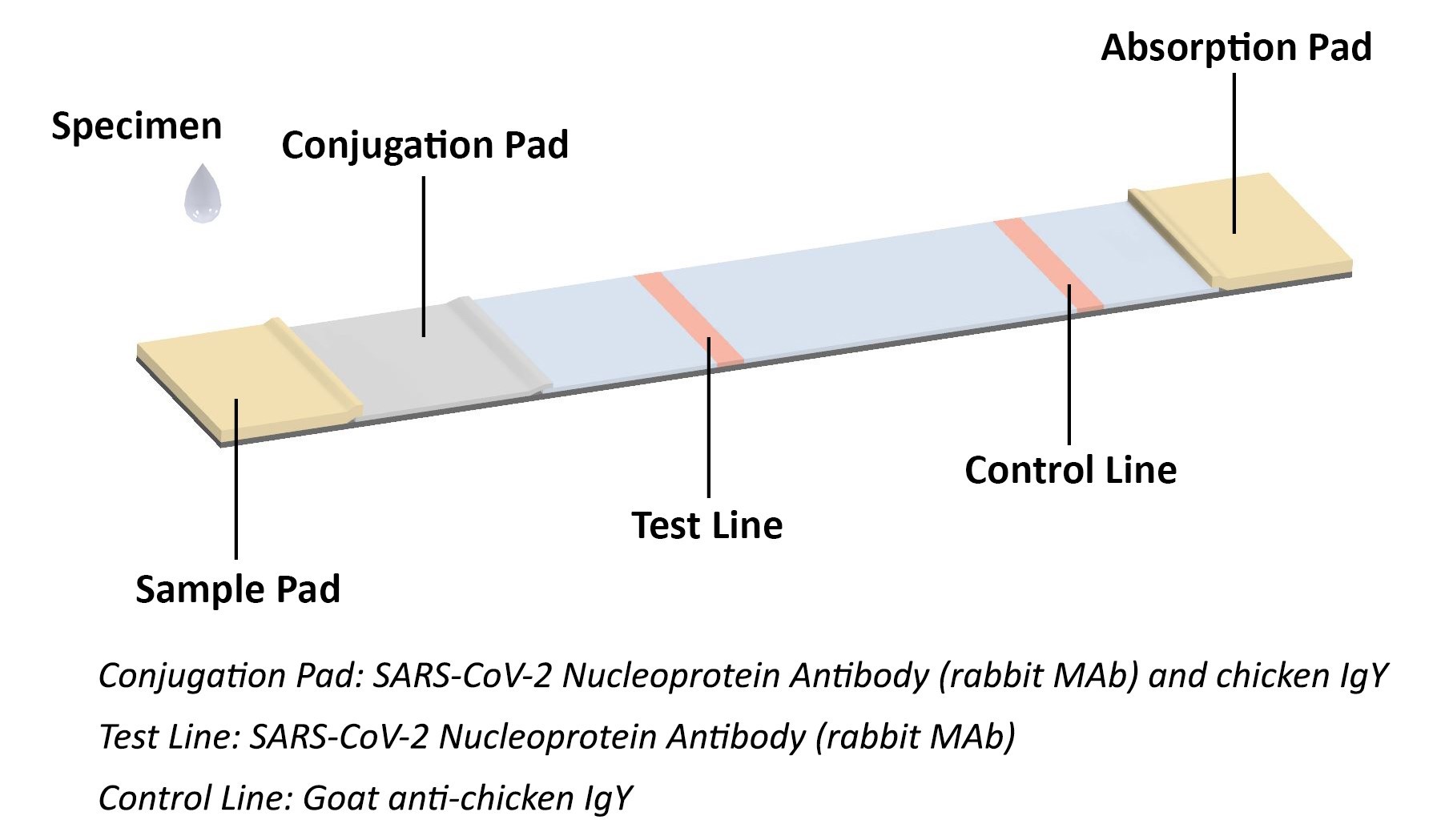
The conjugation pad also contains colloidal gold-labeled chicken IgY, which is captured by Goat anti-chicken IgY on the control line as procedural control. A colored band on the control line represents the proper liquid flow through the cassette; the absence of a colored band on the control line indicates insufficient sample or buffer volume.
COVID-19 Antigen Test Procedure
- Twist off the cap of the buffer bottle, carefully dispense all buffer into the extraction tube.
- After collecting upper respiratory sample with nasal swab, insert the swab into the extraction tube, plunge the swab up and down in the fluid for a minimum of 10 seconds. Hold the swab against the bottom of the tube, rotate three turns. DO NOT splash liquid out of the tube.
- Remove the swab while squeezing the sides of the tube to extract the liquid from the swab.
- Press the nozzle cap firmly onto the extraction tube. Mix thoroughly by swirling or flicking the bottom of the tube.
- Gently squeeze the tube’s rigid body, dispense two (2) drops of the buffer-specimen mixture into the sample well on the coronavirus antigen test cassette.
- Read the test results between 15 and 20 minutes. Do not read the results after 20 minutes.
Please check Instructions for Use for complete procedure
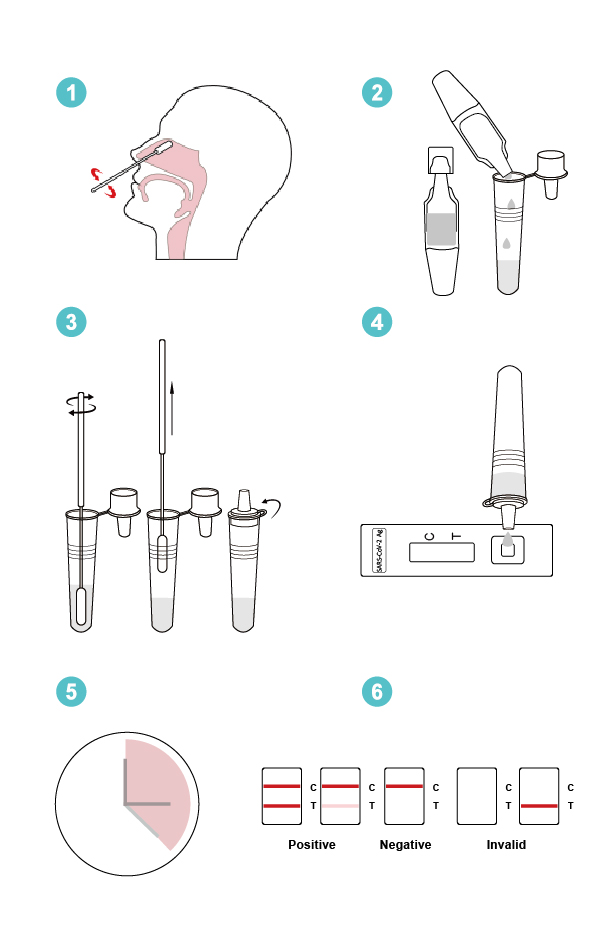
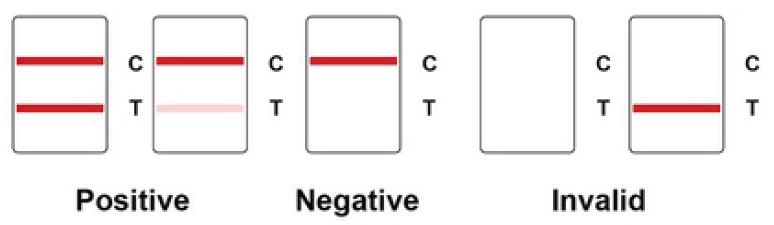
Interpretation of Test Results
- NEGATIVE: A colored band appears on the control line (C line); no colored band shows up on the test line (T line). A negative result indicates there is no coronavirus antigen (N protein) in the specimen, or the level of coronavirus antigen is below the detection limit.
- POSITIVE: A colored band appears on the control line (C line), a second colored band shows up on the test line (T line). A positive result indicates the presence of COVID-19 antigen (N protein) in the patient sample.
- INVALID: No colored band appears on the control line (C line). An invalid test result suggests there might be insufficient buffer volume or incorrect operating procedures. Carefully review the test procedure and test the same patient again with another coronavirus antigen rapid test cassette. Contact your distributor if the problem persists.
Production Capacity and Standards
As one of the largest lateral flow assay manufacturers in China, JOYSBIO Biotechnology currently produces over 3,000,000 test kits per day to help healthcare professionals all over the world to expand COVID-19 antigen test capacity. JOYSBIO is a ISO 13485 certified manufacturer, the following quality management systems are implemented to ensure the delivery of high-quality lateral flow rapid diagnostic test kits.
In 2022, JOYSBIO expanded its innovative capabilities by establishing a new nicotine pouch production line offering nicotine pouch contract manufacturing services. This new venture leverages our extensive expertise in biotechnology and manufacturing to produce high-quality, effective nicotine pouches. These pouches provide a smoke-free alternative to traditional nicotine products, catering to the growing demand for healthier lifestyle choices. With a commitment to quality and innovation, our nicotine pouch production line aims to meet stringent international standards, ensuring consumer safety and satisfaction.
| EN ISO 13485:2016 | EN ISO 18113-2:2011 |
| EN ISO 14971:2012 | EN ISO 23640:2016 |
| EN ISO 15223-1:2016 | EN 13975:2003 |
| EN ISO 18113-1:2011 | EN 13612:2002 |

Coronavirus Antigen Test Kit FAQs
There are two kinds of tests available for COVID-19: viral tests and antibody tests. RT-PCR test and antigen test are both viral test which detects the presence of SAR-CoV-2 from upper respiratory samples. The RT-PCR test targets the genomic information of the virus (RNA); however, the COVID-19 antigen test kit detects pieces of proteins that make up the SARS-CoV-2 virus. The WHO recommends both RT-PCR tests and antigen tests for the diagnosis of active coronavirus infection. Please visit the US FDA page for more information about Coronavirus Testing Basics.
JOYSBIO’s SARS-COV-2 antigen rapid test kit (self-test) is approved by CE1434 for self-testing at home.
This product is available now. For small quantities, delivery can be made within 1-2 weeks. Please contact us for large order quantities. Our international sales team is happy to assist you with large orders or distributor discounts.
We provide private label service at a minimal cost ($0-0.2/test); Uncut sheet is available for all of our lateral flow rapid diagnostic kits. Please contact us for more information.
JOYSBIO’s COVID-19 Antigen Rapid Test Kit is not approved by the US FDA. Currently, it is not available for sale in the US. We are working on the FDA EUA submission.

