PERFORMANCE CHARACTERISTICS
JOYSBIO has validated the COVID-19 IgG/IgM Test Kit by a clinical trial with a total of 400 clinical samples from individual patients: 367 serum samples, and 33 plasma samples (EDTA, heparin, and citrate). The study was performed at provincial hospitals in China from January to April 2020.
Across all study sites, serum and plasma samples from 182 COVID-19 patients with positive Real-Time RT-PCR results, and 218 patients with negative Real-Time RT-PCR results. The evaluation results are shown in the tables below. The sensitivity and specificity of JOYSBIO’s COVID-19 antibody test kit are confirmed to meet diagnosis standards.
- Overall Positive Percent Agreement (Sensitivity, PPA) = 170/182 (93.41%)
- Overall Negative Percent Agreement (Specificity, NPA) = 209/218 (95.87%)
- IgM Sensitivity = 152/182 (83.52%), Specificity = 214/218 (98.17%)
- IgG Sensitivity = 166/182 (91.21%), Specificity = 213/218 (97.71%)
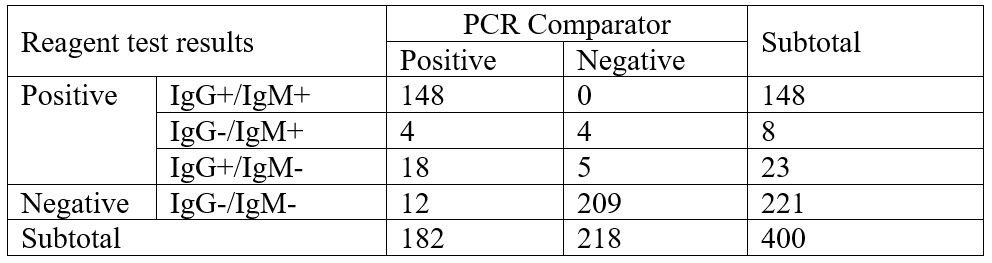
Performance Characteristics Overview
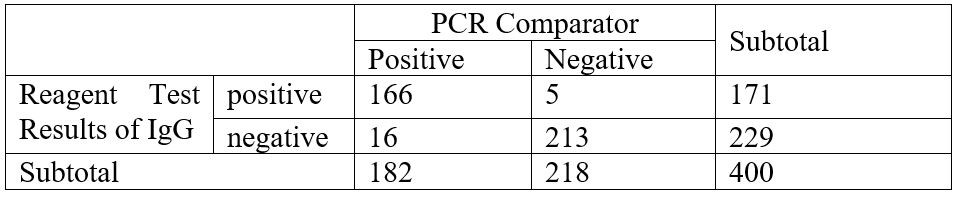
Performance Characteristics- IgG
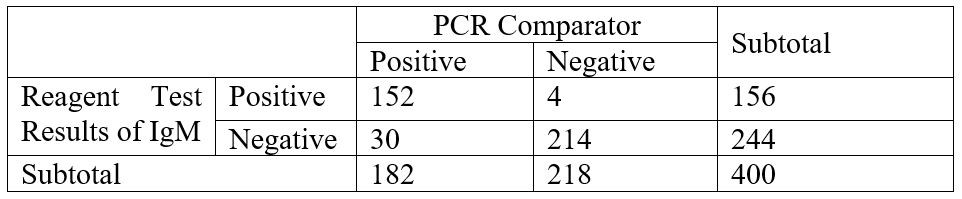
Performance Characteristics- IgM