The emergence of the COVID-19 outbreak has shaken economies of the developed nations and even the health care system. This article explores different techniques being used across the globe for the diagnosis of COVID-19 in humans their principle, test procedure, turn around time, test environment, pros, and cons. Here is a list of COVID-19 test methods that are discussed in this article:
- RT-PCR Based SARS-CoV-2 Detection
- COVID-19 Viral Antigen Test
- COVID-19 Antibody-based Assays
- Lateral Flow Rapid COVID-19 Antibody Test Kit
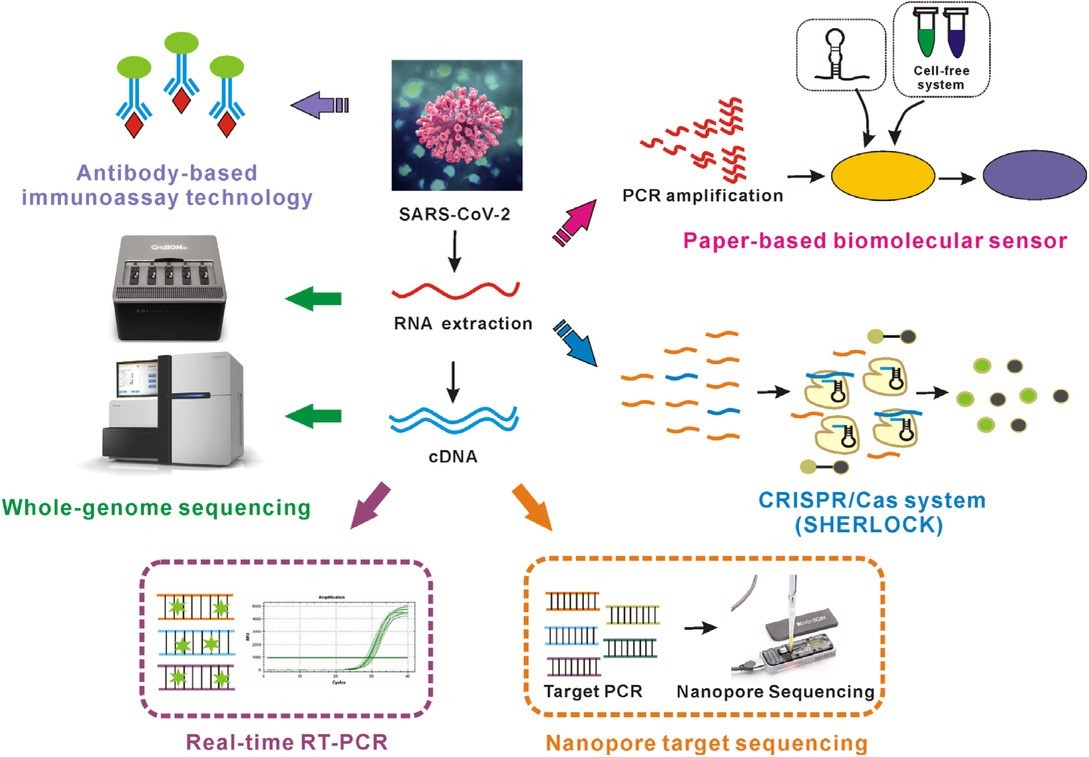
RT-PCR Based SARS-CoV-2 Detection
Real time RT–PCR is one of the COVID-19 test methods which is most generally utilized for distinguishing the COVID-19 active infection. While numerous nations have used continuous RT–PCR for diagnosing different diseases, for example, Ebola and Zika infection, many need support in adjusting this technique for the COVID-19 infection, just as in expanding their national testing limits.
An example is gathered from the pieces of the body where the COVID-19 infection accumulates, for example, an individual’s nose or throat. The case is treated with a few concoction arrangements that evacuate substances, such as proteins and fats, and that extricate just the RNA present in the sample. This extricated RNA is a blend of the individual’s hereditary material and, if present, the infection’s RNA.
The RNA is conversely interpreted to DNA utilizing a particular protein. Researchers at that point include other short sections of DNA integral to explicit pieces of the interpreted viral DNA. On the off chance that the infection is available in an example, these pieces connect themselves to target segments of the viral DNA. A portion of the additional hereditary sections are utilized for building DNA strands during enhancement, while the others are being used for creating the DNA and adding marker names to the strands, which are then used to recognize the infection.
The real time RT–PCR procedure is profoundly touchy and explicit and can convey a dependable determination in as meager as three hours. However, research centers take on average, somewhere in the range of six and eight hours. Contrasted with other accessible infection separation strategies, continuous RT–PCR is essentially quicker and has a lower potential for sullying or mistakes, as the whole procedure can be completed inside a shut cylinder. It keeps on being the most precise strategy accessible for the recognition of the COVID-19 infection.
Be that as it may, real time RT–PCR can’t be utilized to identify past diseases, which is significant for understanding the turn of events and spread of the infection, as infections are just present in the body for a particular window of time. Different techniques are essential to identifying, tracking, and studying past contamination, especially those who may have created and spread without side effects.
COVID-19 Viral Antigen Test
COVID-19 antigen test kits are considered as another COVID-19 test method for diagnosing an active case of COVID-19, particularly early in the infection course, and determining if a patient is contagious to others. By detecting the presence of viral antigen in the nasal, oral, and respiratory tracts, one can determine if a patient is actively shedding virus which can spread to others. These tests are perhaps of most easy-to-operate utility early in the course of infection as they can help confirm viral presence up to 2 days before the onset of symptoms. Given that antibodies may not be detectable until 6-7 days after symptom onset, antigen tests can accelerate the diagnostic window by up to 9 days. The duration of viral shedding can be highly variable and depend on the severity of symptoms, length of illness, and patient-specific immune response. Generally speaking, viral shedding is undetectable 21 to 35 days after symptom onset or 3 to 5 days after a patient becomes asymptomatic.
Therefore, antigen testing has a clear role as an important tool in the diagnostic toolbox for detecting active SARS-CoV-2 infection.
The COVID-19 antigen tests are designed in both ELISA (enzyme-linked immunosorbent assay) and lateral flow rapid-test cassette format. The ELISA test generally gives you a more accurate test result, but the requirement of a laboratory can be a bottleneck01 for developing countries that have limited healthcare resources. A lateral flow assay based COVID-19 antigen test, or antigen rapid test kit, can help with the screening of COVID-19 active infection. Similar to an HIV rapid test cassette, the COVID-19 antigen rapid test kit takes serum, plasma, or fingertip blood and gives a visually interpretable result within 20 mins. Therefore, the antigen test cassette can be a significant help for healthcare professionals to test active SARS-CoV-2 infection at the point of care settings.
COVID-19 Antibody-based Assays
With the rapid acceleration of the COVID-19 pandemic, there has been a rush to develop tests that detect the presence of antibodies produced by the body in response to exposure/infection with the SARS-CoV-2 virus. These testing methodologies rely on the antigen-antibody binding affinity described previously. The test principle relies on a recombinant antigen produced in the laboratory, designed to mimic specific structures of the virus, causing any antibodies present in whole blood or serum with a binding affinity to attach to the antigen. The specificity of the test can be highly dependent on the target antigen chosen as some viral structures can be highly conserved across broad families of viruses, and others can be highly derived and specific to a given strain. Therefore, it is essential to understand the cross-reactivity to other infectious diseases to avoid a false-positive result that may be detecting an antigen from a similar virus, yet not confer immunity to the SARS-CoV-2.
Laboratory-based ELISA antibody assays are read by sensitive laboratory instrumentation, and a controlled aliquot is delivered to the test system, they can be considered quantitative or semiquantitative tests. Therefore, they can determine how much of a given antibody (titer level) are present per unit volume of serum. When operating at the margins of detection, their sensitivity and specificity can be higher than the lateral-flow based device. However, these systems have real disadvantages. Infrastructure costs, requirements for a venous puncture, additional steps in sample processing, time to obtain results, and practical throughput challenges are problematic and substantially increase the cost per test.
In a COVID-19 antibody rapid test (or the lateral flow test kit), antibodies from the serum bind to antigens coated on the test strip and are wicked laterally along the test strip’s length by capillary force. In the indicator regions of the test kit, anti-human antibodies coated on the test strip will bind to form the antigen-antibody complex and hold them in the indicator region. The colloidal gold, or another colorant, accumulates in the indicator region leading to a visible change in color and a narrow band of the wicking substrate. There may be one or more indicator regions with anti-human antibodies specific to IgM, IgG, or other immunoglobulins such as IgA. All tests should also contain a “control” indicator line to confirm that the test sample has appropriately wicked along the assay’s length, and antigens in the test kit remain viable, therefore establishing a correct test procedure. This entire process, from finger-stick to result, occurs in less than 15 minutes.
Lateral Flow Rapid COVID-19 Antibody Test Kit
Most COVID-19 antibody test kits contain indicator lines for both IgM and IgG antibodies, there are several different combinations and corresponding interpretations of results that may occur. Results themselves should always be interpreted by a healthcare professional according to the package insert and instructions for use with the specific test kit being utilized. The following discussion helps link the binary results of the typical antibody test to the clinical meaning and making informed recommendations for patients and the workforce. As discussed earlier, IgM antibodies are produced in the short term after infection, whereas IgG is produced in a more delayed timescale.

The clinical level of functional immunity imparted by SARS-CoV-2 IgG antibodies, and their critical concentration cutoffs, have not been determined. Therefore, recommendations must be based on similar viruses and knowledge of the immune system. For the interpretation of results to make meaningful recommendations on community behavior to test recipients, several factors must be considered:
- Is the subject symptomatic or asymptomatic at the time of antibody testing?
- If symptomatic, how long since symptom onset?
- IgM positive or negative?
- IgG positive or negative?
Interpreting Results When the Subject is Asymptomatic at the Time of Testing
Both IgM and IgG are Negative
The subject’s insusceptible immune system has not delivered any antibodies to the objective viral antigen. On the off chance that it has been more prominent than seven days since the beginning of fever or different indications, the ailment is probably not going to be COVID-19. However, a full-board test, including COVID-19, flu, and bacterial bronchitis, could be performed if accessible and suggested dependent on history and side effects. On the off chance that it has been under seven days since the beginning of side effects, COVID-19 can’t be precluded with immunizer testing alone.
IgG is Positive, and IgM is Negative
The subject’s immune system has produced antibodies to the target viral antigen. The subject is likely in the later stages of the disease course but may still be contagious to others and capable of spreading it. The subject should remain isolated from the disease-negative population for at least 14 days to curtail the chance of spreading the virus. If more rapid return to community work is defensible, for example, for critical industry workers, a molecular test should be performed to assess viral shedding status.
IgM is Positive, and IgG is Negative
The subject’s resistant framework is effectively creating antibodies to ongoing contamination with the objective infection. The issue ought to quickly segregate from reliable people and look for clinical consideration suitable to the manifestation seriousness they experience. The subject can spread the ailment to others now in the malady course. After in any event 14 to 21 days, the question ought to continue testing to decide IgG status before coming back to typical exercises.
Both IgM and IgG are Positive
The subject’s invulnerable framework is effectively creating antibodies to a continuous disease that imaginable started over 14 days back. The question ought to promptly disengage from sound people and look for additional clinical consideration suitable to the side effect seriousness they experience. The subject can almost certainly still spread the infection to other people. Consider continuing testing in 7 to 14 days to affirm IgG just status before coming back to ordinary exercises. On the off chance that progressively quickly go back to the network, activities are justified; for instance, for necessary industry laborers, a sub-atomic test should be performed to survey viral shedding status.
As outlined previously, regardless of the testing methodology used to detect antibodies, these tests are not envisioned as a way to diagnose an active infection. This is primarily because 1) there is a temporal lag between exposure to the virus and the development of antibodies by the immune system, and 2) antibodies will persist long after the body has cleared an infection. These tests are an excellent tool to be used in parallel with molecular testing, patient history, and clinical presentation in symptomatic patients, or used in asymptomatic patients over a period to understand return to activity recommendations. The temporal framework related to exposure, the onset of symptoms, the production of IgM and IgG, symptom resolution, and virus shedding throughout the disease has not been well quantified for SARS-CoV-2 specifically.
The COVID-19 antibody tests can deliver imperative insight to people about their functional immunity to the unending pandemic, giving peace of awareness and supporting with decisions about return to community activities and the workplace. These tests also provide valuable evidence to public health representatives about the binge of the virus in diverse communities, especially in light of the high reported numbers of asymptomatic cases. Furthermore, although never a primary diagnostic tool, antibody status can be used to aid the clinical diagnosis of suspected noncritical cases that present seven days or more after the start of symptoms. In these cases, the use of simple, cost-effective, point-of-care antibody cassette systems can offload the pressure on molecular testing throughput to make those resources more immediately available to the acutely ill and critical patients. The US CDC has not yet concluded if we can use the antibody test result to make public health decisions.
These COVID-19 test methods discussed above are being used across the globe for testing COVID-19 as its ongoing pandemic, but scientists are working to develop more rapid, sensitive, and reliable techniques. These techniques are still under trial, issued as emergency use only during the COVID-19 pandemic.

