Lateral flow assay (LFA) is commonly used in point of care testing because of its great convenience and efficiency. In this article, we are introducing the science behind later flow assays and the applications in the following areas:
- COVID-19 antibody rapid test
- Neutralizing antibody test for COVID-19 vaccine development
- Pregnancy (HCG hormone) test
- The human immunodeficiency viruses (HIV) test
- Hepatitis B virus (HBV) test
What is a lateral flow assay
Lateral flow assay (LFA) is a diagnostic procedure applied to detect and quantify certain analytes present in a complex mixture. The sample analyte, which is to be detected, is kept on a test device to display the results within a time period that ranges between 5 to 30 minutes. The test samples required for the LFAs should mostly be in a liquid state. Due to its easy-to-operate formats, low costs, and short assay times, the lateral flow test kits have found their applications in most fields in which rapid diagnostic tests are required, like hospitals, clinical laboratories, and physicians’ clinics. Apart from being applied in professional medical facilities, these tests have shown significant diagnostic testing convenience at home. It has been suggested that the test sample of around 10 micro-liters could be sufficient to perform the lateral flow assays to determine the sample analytes. The results from the lateral flow assays are generally qualitative, suggesting that it only answers the presence or absence of an analyte. However, the modification in the component materials, reagents, and reader technologies could allow the LFAs to become quantitative and to provide the exact concentration of an analyte in the present blood sample.
Types of Lateral Flow Assays
There are two types of lateral flow assays based on the sample element to be determined. The first and the most common type of LFA is the lateral flow immunoassay (LFIA). The LFIA is further divided into two more categories, out of which one consists of antibodies as the major recognition element, whereas the other one consists of other target recognizing ingredients like proteins, hormones, etc. The other type of LFA includes nucleic acid lateral flow assay, which deals with the detection of amplicons that could be developed during the polymerase chain reaction (PCR). PCR is a technique that is used to amplify a specific DNA sample to a large enough concentration on which further studies could be established.
Principle of lateral flow assays
The principle of lateral flow assays possesses a high level of similarity with that of ELISA. The polymeric strips of the LFA testing devices consist of dry reagents that interact with the target analytes in the fluid sample. The device detects the interaction between the dry reagents and the liquid sample, and the target analyte is detected. The liquid sample that consists of the target analyte moves through various zones of polymeric strips with the help of capillary action. A test strip consists of overlapping membranes mounted on a backing card for better stability and handling.
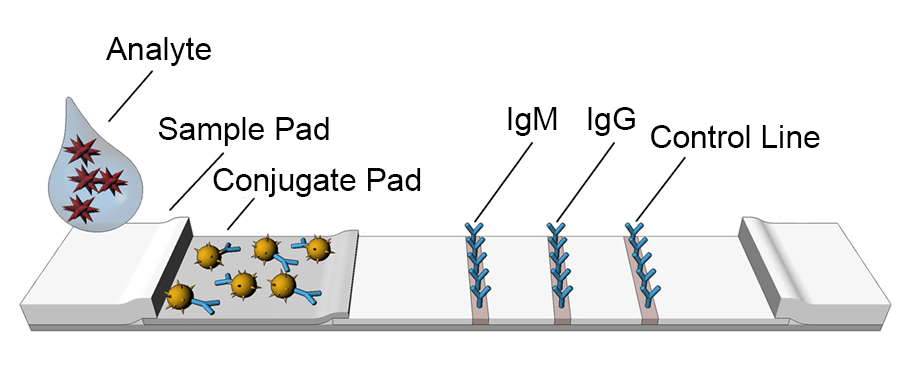
When performing the lateral flow assay, the sample is applied on an adsorbent sample pad, which generally lies on one part of the strip. The sample pad, which is embedded with buffer salts and surfactants, is responsible for the pre-treatment of a sample matrix to make it more suitable for the interaction with the dry reagents. The treated samples are then migrated towards another portion of the strip known as conjugate release pad that consists of antibodies or other substance that binds to the target analyte. After the interaction, the antibody along with target migrates towards a detection zone on LFAs which consists of another set of antibodies or ingredients that reacts to the target molecules that are bonded to the antibodies present in the previous zones. A study from University Medical Centre Groningen shows, the concentration of this compound corresponds to the concentration of the target analyte in samples. If this compound produces a response on the test line, it suggests that the sample contains the target analyte. However, a response at the control line confirms a proper flow of liquid through the strip. The lines appearing at different intensities could be interpreted through eyes or a dedicated reader. Different test lines are on the device if the LFA is intended to test multiple analytes. An adsorbent pad is resent at the end of the strip to avoid backflow and wick excess reagent. The labels are the detecting unit of the sample analyte. Gold nanoparticles are commonly used as a label for the process of lateral flow assay since it fulfills all the requirements.
COVID-19 antibody rapid test kit
The COVID-19 pandemic has affected the definition of everyday lifestyles in almost every country. To fight this pandemic, it becomes quite significant to conduct rigorous testing. The LFA has been considered as one of the most promising techniques considered in the testing of the novel coronavirus. The lateral flow immunoassay is responsible for the detection of antibodies that the body produces when exposed to a foreign body, namely SARS-CoV-2, in this case that causes COVID-19.
The human immune system produces first antibodies “IgM” that have an immediate strength binding to the coronavirus. It has been reported that the IgM antibodies appear in human bodies after approximately five days of a new infection. However, after 8 to 10 days of the COVID-19, the IgG antibodies have a high binding affinity towards the virus, resulting in higher efficiency when fighting the virus. The COVID-19 IgM/IgG antibody test kit qualitatively detects the presence of IgG and IgM antibodies together or separately, thereby studying the immune response of an infected patient. Therefore, the COVID-19 lateral flow assay does not directly detect the SARS-CoV-2 in an infected person but could be used to understand the behavior of the immune response of an affected individual, which could hold a significant level of contribution in combating the COVID-19 global pandemic.
Neutralizing antibody test for COVID-19 vaccine development
Neutralizing antibodies are responsible for preventing viral infections by binding to a cell-free virus and preventing it from infecting the cells. Several studies have suggested that these antibodies could play a significant role in preventing the novel strain of coronavirus. After a SARS-CoV-2 infection, a host produces these neutralizing antibodies to reduce the chances of a recurring infection. It has been reported that the spike protein present in 2019 novel coronavirus, which is a glycoprotein that mediates the invasion of the virus into the human cells, could be used as a target by the neutralizing antibodies. A recent study shows that the COVID-19 vaccine can be proven effective if a high concentration of neutralizing antibodies against the spike protein of SARS-CoV-2 is achieved. The detection of neutralizing antibodies could be an effective method in evaluating the immunity against COVID-19 during the clinical stage and, therefore, play a significant role in testing the efficiency of vaccination against novel coronavirus. Thus, the detection of neutralizing antibodies could help in the development of the vaccination against COVID-19. JOYSBIO launched a lateral flow rapid COVID-19 neutralizing antibody test kit which semi-quantitatively detects the level of neutralizing antibody against SARS-CoV-2 in serum, plasma, and whole blood samples.

LFA application for pregnancy (HCG hormone) test
Rapid detection of the HCG hormone is one of the most convenient techniques in determining early pregnancy. During the early stages of pregnancy, HCG is a hormone responsible for halting the monthly shedding of the inner lining of the uterus to avoid menstrual cycle. Since HCG is a protein, it could easily be detected with the help of the LFIA technique. THE HCG consists of several multigenic sites, which suggest that the antibodies could bind at multiple places to the HCG hormone, direct or sandwich assay applied in the LFIA. The HCG lateral flow assay, or pregnancy test, is used to determine the concentration of HCG in the urine samples.
Rapid test cassettes for the diagnostics of HIV
Another very commonly used commercial application of the lateral flow assay diagnoses Human Immunodeficiency Virus (HIV). The antibodies specific to HIV type 1 are primarily created in response to the HIV-1 subtype B antigens. It has been reported that the application of antigens specific to HIV possesses a better efficiency than the indirect immunoassays. HIV test cassette can capture and detect the reagents by HIV infection by using the multi-valency of the HIV-specific antibodies. This could allow the detection of multiple antibody classes that plays a significant role in the early diagnosis of HIV-1 and HIV-2 antibodies, which could have a considerable impact on the overall efficiency of HIV prevention and treatments.
Lateral flow assays for the detection of Hepatitis Virus
The hepatitis B virus (HBV) has a high global prevalence and is one of the leading causes of preventable death. The HBV point of care (POC) is the most commonly used diagnostic technique used for the HBV. It consists of specific antibodies complementary to the HBV. These assays are generally quantitative, and the readouts’ results are binary, which includes only the absence and presence of the HBV. Although the LFIAs are qualitative, studies have revealed that it has high sensitivity, which decreases the occurrence of false positives and false negatives, thereby possessing a high accuracy. The human serum or plasma, which is the whole blood, could be used to perform the rapid testing for the hepatitis C virus (HCV). The lateral flow tests also possess high sensitivity for the detection of HCV.

