COVID-19 Neutralizing Antibody Test Kit
COVID-19 Neutralizing Antibody Test Kit
On July 4, 2020, JOYSBIO launched the first rapid COVID-19 Neutralizing Antibody Test Kit (lateral flow cassette) for detection of SARS-COV-2 neutralizing antibody (NAb), which can be used to determine the immunity status after infection or vaccination.
Similar to many infectious diseases, neutralizing antibodies can help to inhibit SARS-CoV-2 replication, which means the level of neutralizing antibody correlates to the immunity of future SARS-CoV-2 infections. A rapid detection of neutralizing antibodies can help with vaccine development, plasma therapy, and immunology study.
Features:
- Rapid immunity evaluation within 25 mins.
- Proved by clinical evaluation with subjects who received Pfizer-BioNTech vaccine.
- Compatible with serum, plasma, whole blood, and fingertip blood.
- Compatible with optical reading device for POC.
What are Neutralizing Antibodies
Not all the antibodies are neutralizing. Non-neutralizing antibodies, or binding antibodies, are able to bind to viral antigens but do not block viral infection. Binding antibodies can flag the viral antigen to trigger immune responses but the presence of binding antibodies does not reflect the level of immunity. Neutralizing antibodies (NAbs) are antibodies that not only bind to viral antigens, but also block viral infection. The presence of NAb can be used to evaluate immunity status after infection or vaccination.
Neutralizing Antibody Rapid Test Intended Use
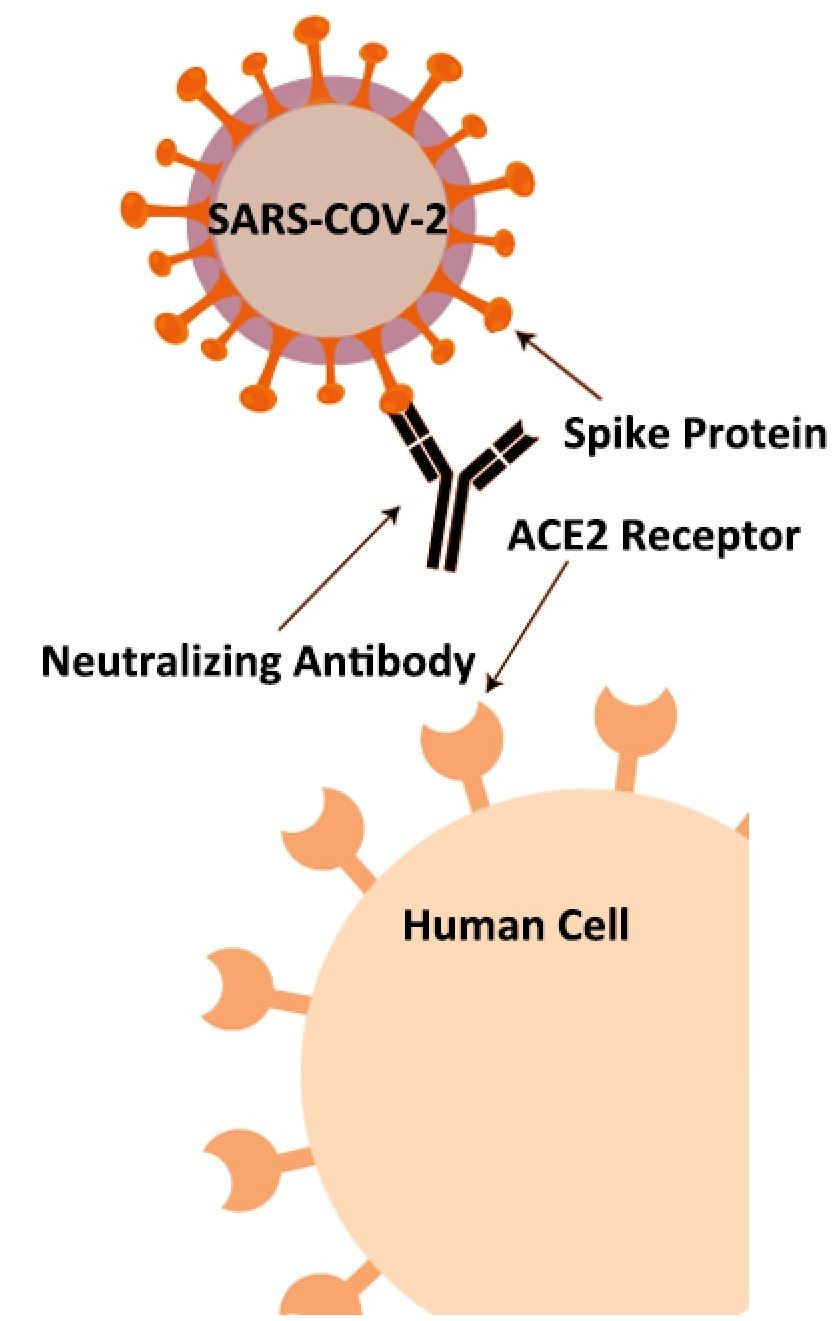
The 2019 novel coronavirus (SARS-CoV-2) has several structural proteins, including spike (S), envelope (E), membrane (M), and nucleocapsid (N). The S-protein contains a receptor binding domain (RBD), which can recognize the cell surface receptor, angiotensin-converting enzyme-2 (ACE2). In a recent study, neutralizing antibody (NAb) can block the interaction between the receptor-binding domain (RBD) of the novel coronavirus spike protein with the ACE2 cell surface receptor. The level of NAb, therefore, can be used to analyze a patient’s immunity against future SARS-CoV-2 infection. This COVID-19 neutralizing antibody lateral flow assay rapidly detects any antibodies that can neutralize the RBD-ACE2 interaction.
Researchers have been using traditional viral neutralization assay for testing SARS-CoV-2 neutralizing antibodies and there are several ELISA based neutralizing antibody test kits available in the market since May 2020. However, performing COVID-19 neutralization assays or ELISA requires complex laboratory settings, and it is time-consuming, despite a higher sensitivity and specificity. JOYSBIO’s surrogate neutralizing antibody rapid test cassette provides an easy way for the preliminary screening of NAb to estimate patients’ immunity to novel coronavirus infection.
COVID-19 NAb Rapid Test Principle
JOYSBIO’s COVID-19 neutralizing antibody test kit is a lateral flow assay for rapid NAb screening, which can mimics the virus neutralization process. This lateral flow assay contains two key components: the recombinant SARS-CoV-2 RBD fragment and chicken IgY, labeled by colloidal gold are as tracers; and the human ACE2 receptor protein (hACE2) and goat anti-chicken IgY antibody, coated with cellulose nitrate membrane. The protein-protein interaction between RBD and hACE2 can be blocked if the test samples contains a certain level of neutralizing antibody against SARS-CoV-2.
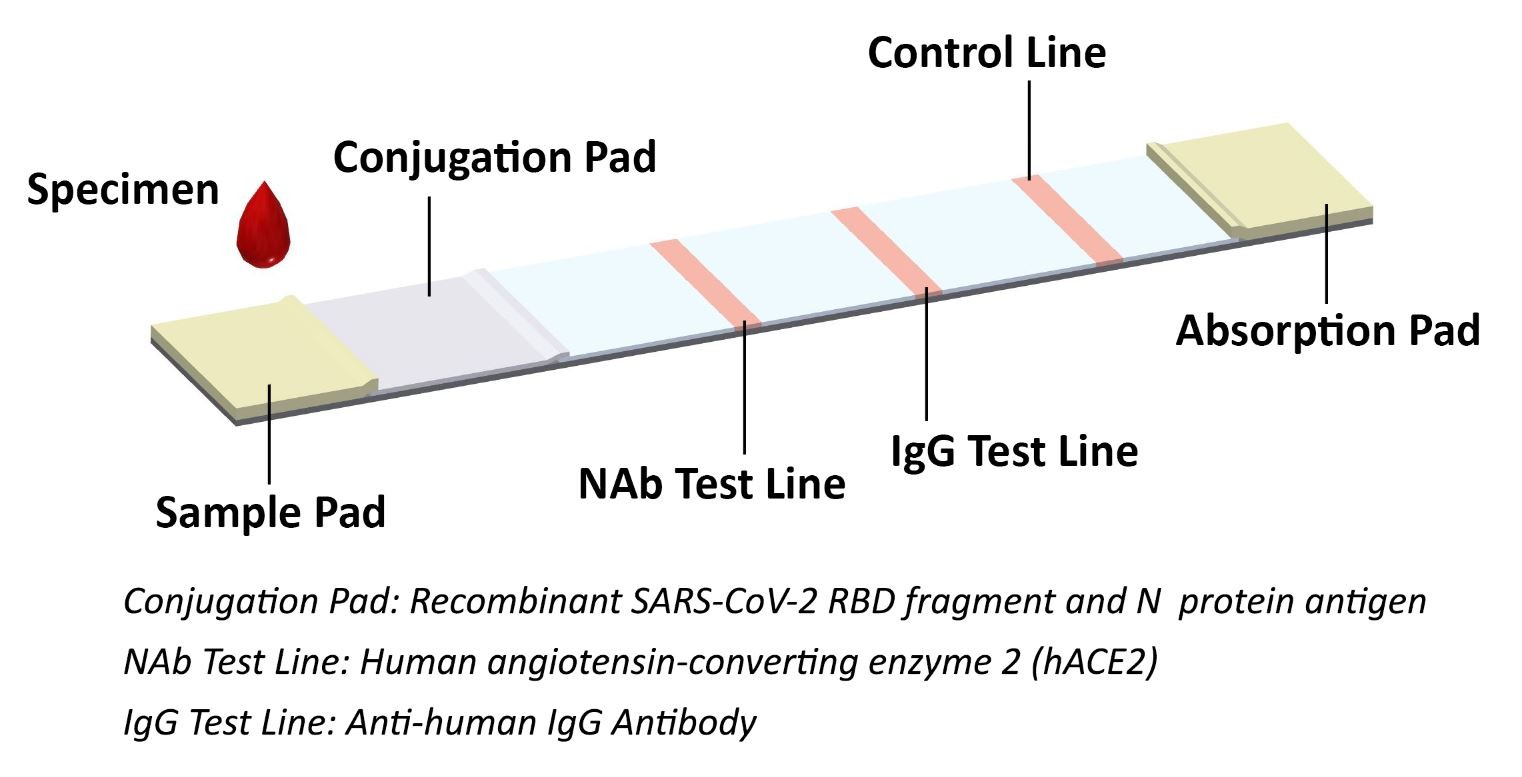
When specimens are added to the sample pad, neutralizing antibodies present in the specimen will bind to the RBD labeled colloidal gold and block the protein-protein interaction between RBD and hACE2. The unbound RBD labeled colloidal gold as well as any RBD labeled colloidal gold bound to non-neutralizing antibody will be captured on the test line. The colloidal gold labeled chicken IgY antibody is bound to the goat anti-chicken IgY antibody coated with a colored band (C line), which acts as a procedural quality control line.
Clinical Evaluation
The clinical sensitivity of the kit is determined by specimens collected from 93 participants who received Pfizer-BioNTech COVID-19 mRNA vaccine (Tozinameran or BNT162b2) in Italy between January 2021 and March 2021. The clinical specificity of the kit is determined by specimens from 317 uninfected and unvaccinated participants from Heilongjiang Province Hospital in China. The reference reagent used in the clinical study is cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit manufactured by GenScript USA Inc. The kit showed 92.47% of sensitivity and 99.68% of specificity.
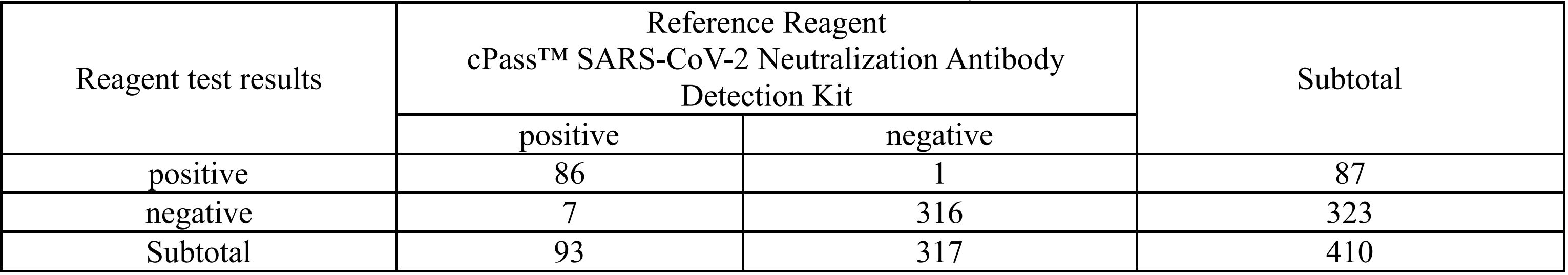
- Positive Percent Agreement (PPA)= 86/93(92.47%) (95%CI: 85.1%-96.9%)
- Negative Percent Agreement: (NPA) = 316/317 (99.68%) (95%CI: 98.2%-100.0%)
- Accuracy= (86+316)/410×100%= 98.05%
- Kappa= 2×27169/284034= 0.94>0.5
COVID-19 Neutralizing Antibody Rapid Test Operation Procedure
*Please see product instruction for use (IFU) for detailed operation procedure.
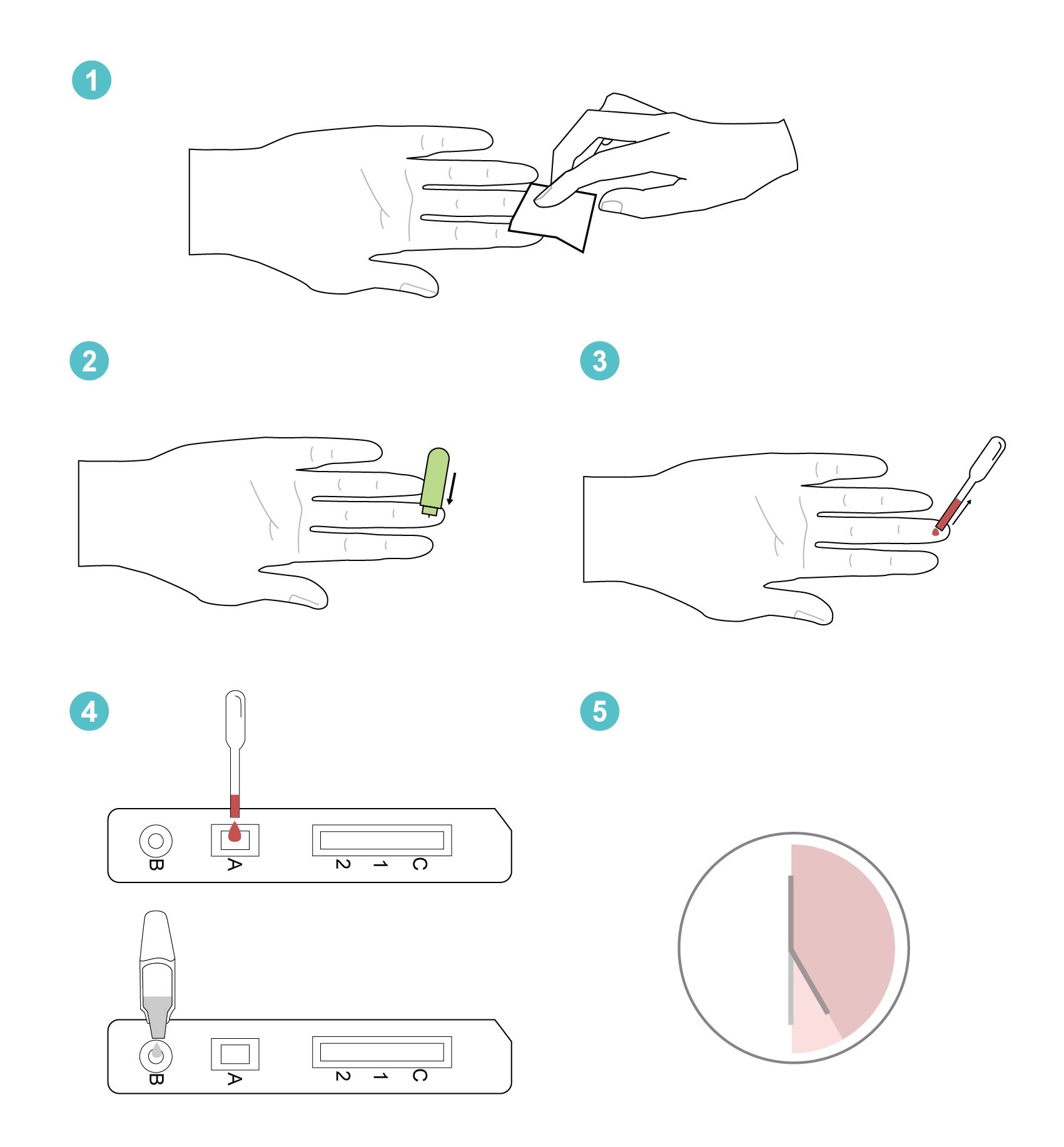
Step 1:
Wipe to clean the puncture site on your finger with the alcohol pad.
Step 2:
Remove the cap from safety lancet, push the lancet firmly against the puncture site.
Step 3:
Use the disposable pipette to draw the blood from puncture site.
Step 4:
Add one (1) drop (20µL) of blood from disposable pipette to sample well on the test cassette.
Add three (3) drops (100µL) of dilution buffer from the buffer bottle to the buffer well on the test cassette.
Step 6:
Start the timer. Read the test results between 25 and 30 minutes. Do not read the results after 30 minutes
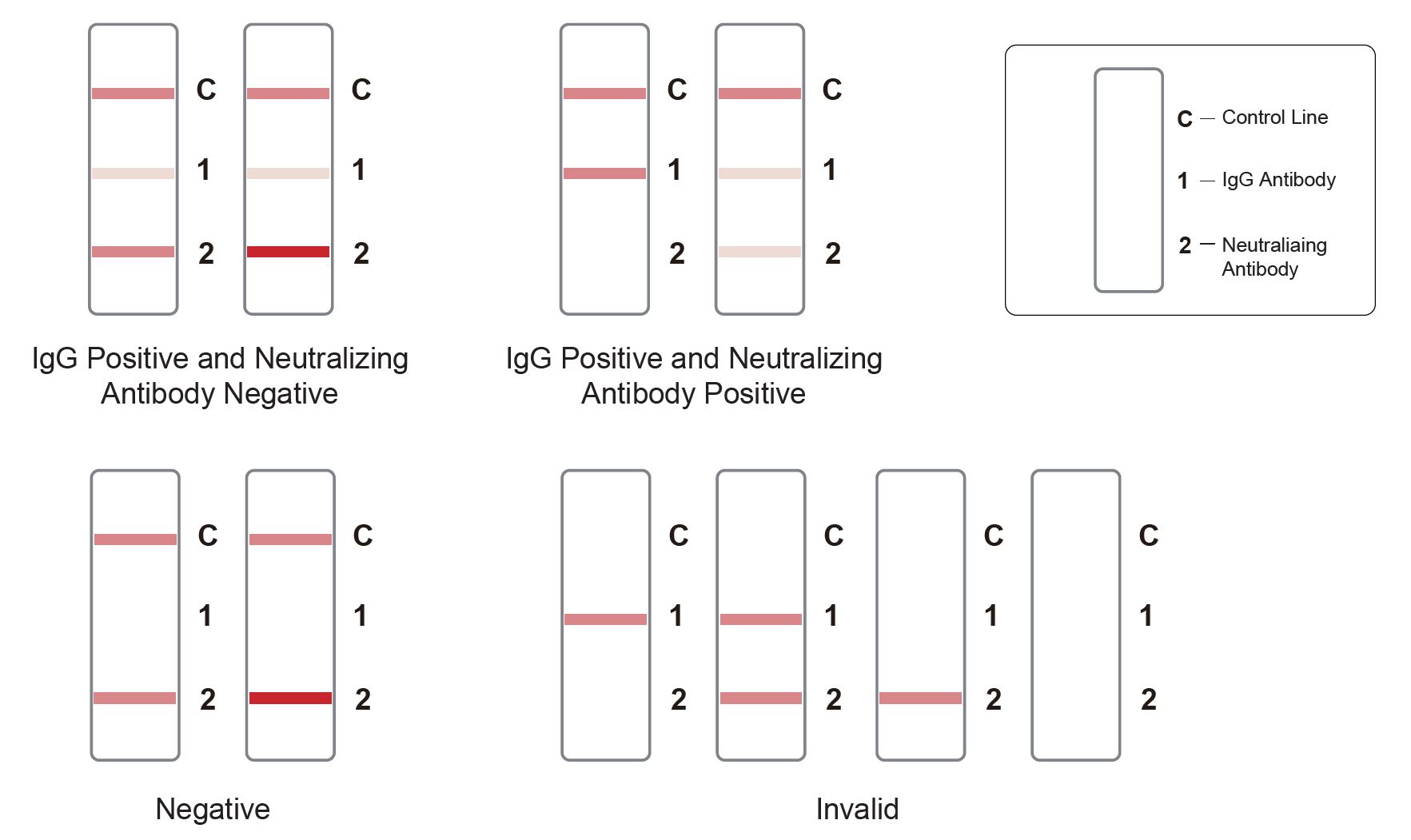
Result Interpretation
WIthout a cassette reading device, test results can be interpreted visually:
- IgG Positive and Neutralizing antibody Negative:Three lines appear. One colored line appears in the control line region (C), a colored line appears in IgG test line (1) region, and a colored line appears in neutralizing antibody test line (2) region which is darker or equal than C line.
- IgG Positive and Neutralizing antibody Positive: One colored line appears in the control line region (C), a colored line appears in IgG test line (1) region, and the color in neutralizing antibody test line (2) region is lighter than C line. When the neutralizing antibody test line (2) region does not show a visible line, it represents a high neutralizing antibody level.
- NEGATIVE: Two lines appear. One colored line in the control line region (C), and a colored line appears in Neutralizing antibody test line (2) region which is darker or equal than C line.
- INVALID: Control line fails to appear.